April 27 – May 1
Monday: Review MC portion of practice test, Review FR portion of practice test and from 2012-2013 packet
HOMEWORK: review topics you did not understand
Tuesday: Review MC portion of practice test, Review FR portion of practice test and from 2012-2013 packet
HOMEWORK: review topics you did not understand
Wednesday: Take Aqueous Equilibria Test
HOMEWORK: review topics you did not understand
Thursday: Review Quiz over multiple choice packet
HOMEWORK: review topics you did not understand
Friday: Review Quiz over one of the packet free response
HOMEWORK: Relax and know that there is little you can do at this point!
May 4-8
Monday: AP Chemistry test, 8 AM. I will have a continental breakfast for you starting at 7:20.
Tuesday – Friday: LAB: Separation and Qualitative Determination of Cations and Anions
Do you know your cations and anions lab
HOMEWORK: Lab due Tuesday, May 12 (seniors) and Thursday, May 14 (juniors). Bring in 100% cotton t-shirt, zippered bag, and $1 by Monday, May 11.; take SLO
May 11 – 15
Monday First half of period: LAB: Separation and Qual. Determination of Cations and Anions, Take SLO
Second half of period: Tie up shirts and soak for tie dye Student Tie Dye Instructions
HOMEWORK: Lab due Tuesday, May 12 (seniors) and Thursday, May 14 (juniors).
Tuesday First half of period: LAB: Separation and Qual. Determination of Cations and Anions
Second half of period: Tie up shirts and soak for tie dye
HOMEWORK: Lab due Tuesday, May 12 (seniors) and Thursday, May 14 (juniors).
Wednesday: Tie Dye (lab will only be available after school)
HOMEWORK: Lab due Tuesday, May 12 (seniors) and Thursday, May 14 (juniors).
Thursday: Dye shirts for tie dye (lab is not available today)
HOMEWORK: Study for final exam (non-AP test takers only)
Friday: Final exam for non-AP test takers. Multiple Choice portion: 33 questions.
HOMEWORK: Study for final exam
May 18 – 22
Monday: Final exam for non-AP test takers. Free Response portion: 4 multi-part questions.
HOMEWORK: Study for exam
Tuesday: nothing going on. All exams will be given.
HOMEWORK: Study for exam
Wednesday: 7th period exam…nothing going on. All exams will be given.
Thursday: 2, 4, 6 period exams
Friday: 1, 3, 5 period exams
============================
APPLICATIONS OF AQUEOUS EQUILIBRIA
Chapter 15
Topics to Know
Common Ion Effect, Buffers, Buffered Solutions, Titrations, Titration Curves, Indicators, Ksp
Concepts to Know
Recall the definition of a buffer
Understand and how a buffer works
Be able to identify and calculate the pH of a buffer solution
Understand the techniques and procedures associated with titrations
Be able to sketch titration curves and be able to suggest a suitable indicator for a particular titration
Understand the hydrolysis of salts and the effect this has on pH
Understand the meaning of the term ‘equivalence point’
Understand how indicators work
To Do
Read Chapter 15, pages 681-739
Ch. 15 Problem set (due April 17): 23, 25, 33, 35, 37, 39, 45, 51, 55, 65, 77, 81, 91, 97
Videos to Watch
The Common Ion Effect: https://www.youtube.com/watch?v=xUtSIDu8-6M&list=PLZbtLo_n3SRZqrj_Ewct-BbvWK2PeSwhk
Calculating the pH of strong acids and strong bases: https://www.youtube.com/watch?v= bGtOLRnGNy8&list=PLA612E669DD96FAE4
Calculate the pH of a weak acid: https://www.youtube.com/watch?v=eYa_xpzxToE&list= PLA612E669DD96FAE4
Calculate the pH of a weak base: https://www.youtube.com/watch?v=VdlJ1x4Gfl0&list= PLA612E669DD96FAE4
Titration Curve: https://www.youtube.com/watch?v=L6N6HOekQck&list=PLZbtLo_n3SRZqrj_Ewct-BbvWK2PeSwhk
The Solubility Equilibria of Salts: https://www.youtube.com/watch?v=3RJQvLwNU5g&list= PLZbtLo_n3SRZqrj_Ewct-BbvWK2PeSwhk
Schedule of Topics – REVISED x2
April 13-17
Monday: Equations Quiz; Common Ion Effect, Buffered Solutions
Notes: Aqueous Equilibria notes
PowerPoint for all notes: Aqueous EQ – web
HOMEWORK: Buffered Solutions Buffered Solns
Tuesday: Buffering Capacity, Titration Curves
HOMEWORK: Acid and Base Titration Acid and Base Titration
Wednesday: Titration Curves, Indicators, Ksp
HOMEWORK: Titration and Ksp Titration and Ksp
Thursday: LAB: Preparation of a Buffered Solution Buffered Solution lab
HOMEWORK: Lab due Wednesday, April 30
Friday: Catch up notes day
HOMEWORK: whatever we have not gotten to.
April 20-21
Monday: , Catch up notes day
HOMEWORK: Acid and Base Titration Acid and Base Titration
Tuesday: Catch up notes day, work on lab
HOMEWORK: ration and Ksp Titration and Ksp
Wednesday: SLO
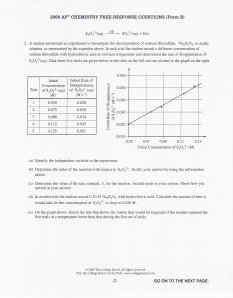
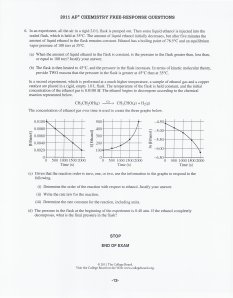
HOMEWORK: Aqueous Equilibria Free Response Aqueous EQ Free Response
Tuesday: Homework Check over problem set, Review for test, work on lab Aqueous Equilibria Review
HOMEWORK: Study for test
Friday: Applications of Aqueous Equilibrium Test
HOMEWORK: Start going through your index cards and Free Response packet.
====================================
ACIDS AND BASES
Chapter 14
Topics to Know
Arrhenius and Bronsted-Lowery definitions, Conjugate acid and base pairs, strong and weak acids, Ka. Kb. Kw, pH, pOH, [H+], [OH ], percent dissociation, polyprotic acids, acid-base reactions.
Concepts to Know
• Know and apply Arrhenius and Bronsted-Lowry Definitions of acids and bases and apply on specific examples.
• Recognize acids and bases in reactions and identify the conjugate partner of each.
• Recognize common monoprotic and polyprotic acids and bases and write balanced equations for their ionization in water.
• Understand and explain on the molecular bases what makes some acids strong while others weak; all acids and oxyacids.
• Define acid/conjugate base pairs in a reaction as well as write formulas for conjugate acids and conjugate bases of specific compounds or ions.
• Know and apply definitions of amphiprotic and amphoteric.
• Understand the concept of water autoionization, what the hydronium ion (H3O+) represents. Use the water ionization constant, Kw.
• Identify if a compound or ion is a strong or weak acid or base.
• Write equilibrum constant expressions for weak acids and weak bases.
• Understand that weak acids can be molecules (like acetic acid), cations (NH4+) or hydrated metal cations [Fe(H2O)6]3+ or anions (HCO3-).
• Understand the relationship between Ka for a weak acid and Kb for its conjugate base.
• Write hydrolysis reactions for any acid, base (strong or weak) or to show that a specific ion or compound is an acid and/or base.
• Write equations for acid-base reactions and decide whether they are product favored or reactant-favored.
• Predict if a solution of a compound/salt/ion is acidic or basic.
• Calculate the [H+], [OH-], pH and pOH of any solution containing a weak or strong acid or base (including salts).
• Calculate Ka or Kb of any species (usually the conjugate acid or conjugate base) using Kw.
• Use pH, %ionization and concentration of a compound or ion to calculate pH, pOH, Ka, and/or Kb of this species.
• Calculate the pH of a mixture of weak acid (or weak base) and its conjugate base (or conjugate acid).
• Calculate the [H+], [OH-], pH and pOH after know volumes of known concentrations (therefore known moles) of strong acids and strong bases are mixed, strong acids and weak bases are mixed or weak acids and strong bases are mixed.
• Calculate the pH of a solution of a polyprotic acid or base.
To Do
Read Chapter 14, pages 623-672
Ch. 14 Problem set (due April 3): 28, 29, 33, 43, 47, 53, 61, 63, 65, 77, 79, 85, 95, 103
Videos to Watch
Acids and Bases Intro, Part One: https://www.youtube.com/watch?v=KasfDV04Xsg&list= PLZbtLo_n3SRZWaitNwCawMq7pnf1FHswV
Acids and Bases Intro, Part Two: https://www.youtube.com/watch?v=T2_OjEiRPFQ&list= PLZbtLo_n3SRZWaitNwCawMq7pnf1FHswV
Weak Acid ICE tables: https://www.youtube.com/watch?v=T2_OjEiRPFQ&list= PLZbtLo_n3SRZWaitNwCawMq7pnf1FHswV
Percent Dissociation: https://www.youtube.com/watch?v=vqs9GEIsytg&list= PLZbtLo_n3SRZWaitNwCawMq7pnf1FHswV
Weak Bases ICE tables: https://www.youtube.com/watch?v=MaZ73HvwAHM&list= PLZbtLo_n3SRZWaitNwCawMq7pnf1FHswV
Polyprotic Acids: https://www.youtube.com/watch?v=4hlyXMMYORc&list= PLZbtLo_n3SRZWaitNwCawMq7pnf1FHswV
Properties of Salts: https://www.youtube.com/watch?v=DQHEW0IFJxM&list= PLZbtLo_n3SRZWaitNwCawMq7pnf1FHswV
Schedule of Topics
March 20
Friday: Acids and Bases Basics
Notes: Acids and Bases Notes
Powerpoint of all notes: Acids and Bases – Web
HOMEWORK: Acid and Base Free Response I Acid and Base FR I
March 24-27
Tuesday: Ka. Kb. Kw, pH, pOH, [H+], [OH ], percent dissociation
HOMEWORK: pH, pOH, [H+], [OH ], weak acids and bases pH Ka Handout
Wednesday: LAB: Determination of Ka of Weak Acids Determination of Ka
HOMEWORK: Lab due Thursday, April 2
Thursday: More on Ka and Kb, percent dissociation, polyprotic acids
HOMEWORK: Acid and Base Free Response II Acid and Base FR II
Friday: Homework Check over Problem set,
HOMEWORK: Acid and Base Multiple Choice
March 30 – April 3
Monday: Review for test Acids and Bases Review
HOMEWORK: Study for test
Tuesday: Acids and Bases Test
HOMEWORK: Look over equations packet
Wednesday: Equations Review
HOMEWORK: Study equations quiz
Thursday: Equations Review
HOMEWORK: Study for equations quiz
Friday: Equation Quiz
HOMEWORK: Look over chapter 15, Applications of Aqueous Solutions
=================================
KINETICS
Topics to Know
Collision theory, factors affecting the rate of a reaction, Maxwell Boltzman Distribution plot, energy profile plot, average reaction rate, differential rate law, integrated rate law, reaction mechanisms, catalysts.
Concepts to Know
- Be able to recall AND understand Collision Theory
- Be able to recall AND understand how temperature, concentration, surface area and catalysts affect a rate of reaction
- Understand AND be able to interpret a Maxwell-Boltzman distribution plot
- Understand AND be able to interpret an energy profile plot
- Be able to deduce orders, rate equations and rate constants (including units) from initial rate data
- Understand the link between the rate determining (slow step) in a reaction mechanism and the rate equation
- Understand AND be able to interpret graphical data relating to rates
To Do
Read Chapter 12, pages 527-566
Ch. 12 Problem set (due March 19): 11, 25, 27, 29, 31, 32, 33, 51
Videos to Watch
Kinetics Basics: https://www.youtube.com/watch?v=yZ6uDU8sKLM&list= PLZbtLo_n3SRZyER3WMJ_y8-w3bBGeT4jt
Differential Rate Law I: https://www.youtube.com/watch?v=Sddo8qAScxM&list= PLZbtLo_n3SRZyER3WMJ_y8-w3bBGeT4jt
Differential Rate Law II: https://www.youtube.com/watch?v=Sddo8qAScxM&list= PLZbtLo_n3SRZyER3WMJ_y8-w3bBGeT4jt
Integrated Rate Law: https://www.youtube.com/watch?v=MeS4DOFP9Zo&list= PLZbtLo_n3SRZyER3WMJ_y8-w3bBGeT4jt
Reaction Mechanisms: https://www.youtube.com/watch?v=uWbBciv3xBg&list= PLZbtLo_n3SRZyER3WMJ_y8-w3bBGeT4jt
Model for Kinetics: https://www.youtube.com/watch?v=zVsWEqzQMgs&list= PLZbtLo_n3SRZyER3WMJ_y8-w3bBGeT4jt
Catalysis and Potential Energy: https://www.youtube.com/watch?v=1yKDPAA7MA4&list= PLZbtLo_n3SRZyER3WMJ_y8-w3bBGeT4jt
Schedule of Topics
March 11-13
Wednesday: Chemical Reaction Rates, Differential Rate Law
The Kinetics notes packet: Kinetics Notes 2015
Powerpoint of lectures: Kinetics – web
HOMEWORK: Kinetics Free Response I Kinetics FR I
Thursday: Practice (Kinetics Free Response II Kinetics FR II), Integrated Rate Law
HOMEWORK: Kinetics Free Response III
Friday: Professional Talk with graduate chemist
March 16-20
Monday: Reaction Mechanisms, Catalysis
HOMEWORK: Kinetics Free Response IV Kinetics FR IV
Tuesday: LAB: Kinetics: Experimental Determination of Rate LawThe Iodine Clock Reaction – AP
HOMEWORK: Lab is due Wednesday, March 25
Wednesday: LAB: Kinetics: Experimental Determination of Rate Law
HOMEWORK: Lab is due Wednesday, March 25
Thursday: Homework check over problem set, Review for test Kinetics Review
HOMEWORK: Complete Review packet , study for test
OPTIONAL HOMEWORK: Kinetics Free Response V
Monday: Kinetics test
HOMEWORK: next chapter – Acids and Bases
=====================================
THERMODYNAMICS
Topics to Know
Enthalpy, Entropy, spontaneous reaction, Second law of Thermodynamics, Third Law of Thermodynamics, Free Energy
Concepts to Know
- Understand and be able to apply the concept of entropy both in descriptive and calculation contexts.
- Understand and be able to apply the concept of Gibbs free energy both in descriptive and calculation contexts.
- Know the relationship of change in free energy to equilibrium constants and electrode Potentials
To Do
Read Chapter 16, pages 748-781
Ch. 16 Problem set (due March 11): 24, 25, 29, 33, 37, 39, 45, 51, 57, 62
Videos to Watch
Thermodynamics I, Enthalpy and Entropy: https://www.youtube.com/watch?v=dRg2s2X8ed4&list =PLP24P3yfORxDvl6raS5_8u409SQGFif5K
Thermodynamics II, Enthalpy and Entropy: https://www.youtube.com/watch?v= XUzn15CKvEs&list=PLP24P3yfORxDvl6raS5_8u409SQGFif5K
Thermodynamics III, Enthalpy and Entropy: https://www.youtube.com/watch?v= HdGu1WPP9mY&list=PLP24P3yfORxDvl6raS5_8u409SQGFif5K
Thermodynamics IV, Enthalpy and Entropy: https://www.youtube.com/watch?v= k4EMwiByd80&list=PLP24P3yfORxDvl6raS5_8u409SQGFif5K
Thermodynamics 2, Thermodynamic Calculations I: https://www.youtube.com/watch?v= p898BLj6SXI&list=PLP24P3yfORxDvl6raS5_8u409SQGFif5K
Thermodynamics 2, Thermodynamic Calculations II: https://www.youtube.com/watch?v= S0U6aMRw81M&list=PLP24P3yfORxDvl6raS5_8u409SQGFif5K
Schedule of Topics
March 2-6
Monday: Entropy
Powerpoint: Thermodynamics – web
Notes: Thermodynamics Notes
HOMEWORK: Thermodynamics Free Response I Thermodynamics FR I
Tuesday: Gibbs Free Energy
HOMEWORK: Entropy, Enthalpy, and Free Energy Entropy and Free Energy
Wednesday: Putting it all together
HOMEWORK: Thermodynamics Free Response II Thermodynamics FR II
Thursday: LAB: Thermodynamics – Enthalpy of Reaction and Hess’ Law Thermodynamics – enthalpy and Hess Lab
HOMEWORK: Lab is due Friday, March 13
Friday: LAB: Thermodynamics – Enthalpy of Reaction and Hess’ Law
HOMEWORK: Lab is due Friday, March 13
March 9-10
Monday: Homework check over problem set, Review for test
HOMEWORK: Complete review packet, study for test.
OPTIONAL: Thermodynamics Free Response III Thermodynamics FR III
Equations Packet: Equation Packet – Brim-Meriwether
Tuesday: Thermodynamics test
HOMEWORK: next chapter – Kinetics; start reviewing equations
====================================
THERMOCHEMISTRY
Topics to Know
Potential energy, kinetic energy, Enthalpy, energy and work, First law of Thermodynamics, state function, balancing thermochemical equations, Hess’s Law
Concepts to Know
- Differentiate between potential energy and kinetic energy.
- Explain what is meant by a system and its surroundings.
- State the 1st Law of Thermodynamics.
- Apply the 1st Law of Thermodynamics quantitatively.
- Explain what is meant by a state function.
- Describe enthalpy and balance thermochemical equations.
- Calculate enthalpy changes in physical and chemical processes.
- Use Hess’s Law to calculate heat of reaction for multi-step reactions.
- Write out heat of formation equations and use heat of formation to calculate heat of reaction.
To Do
Read Chapter 6, pages 228-265
Ch. 6 Problem set (due February 28): 21, 26, 29, 35, 38, 51, 57, 59, 61, 67, 71
Videos to Watch
Energy, Work, and Heat: https://www.youtube.com/watch?v=-imKJkXuF8w&list=PLZbtLo_n3SRYyKKMV-s-pLugpw6MATG3o
Calorimetry, Enthalpy, Energy: https://www.youtube.com/watch?v=jGc_bPHQH1I&list= PLZbtLo_n3SRYyKKMV-s-pLugpw6MATG3o
Heat Capacity: https://www.youtube.com/watch?v=kXO7NO24ulQ&list= PLZbtLo_n3SRYyKKMV-s-pLugpw6MATG3o
Thermochemical Stoichiometry: https://www.youtube.com/watch?v=qo3mj_U5RL4&list= PLZbtLo_n3SRYyKKMV-s-pLugpw6MATG3o
Hess’s Law: https://www.youtube.com/watch?v=CMJedVhIJ1w&list= PLZbtLo_n3SRYyKKMV-s-pLugpw6MATG3o
Heat of Formation: https://www.youtube.com/watch?v=vZmOw3rhhAc&list= PLZbtLo_n3SRYyKKMV-s-pLugpw6MATG3o
Schedule of Topics
February 12-13
Thursday: Energy, Work, and Heat
HOMEWORK: Energy, Work, and Heat Energy Work Heat
Friday: Enthalpy
HOMEWORK: Enthalpy Enthalpy
February 17-20
Tuesday: SNOW DAY
Wednesday: Bond Energies
HOMEWORK: work on problem set, read through lab
Thursday: The Hand Warmer Challenge: Where does the heat come from? Hand Warmer lab
HOMEWORK: Lab due Friday, February 27
Friday: The Hand Warmer Challenge: Where does the heat come from?
HOMEWORK: Lab due Friday, February 27
February 23-24
Monday: Homework check of problem set
HOMEWORK: Thermochemistry Practice Thermochem Practice
Tuesday: Review for test
HOMEWORK: Study for test Thermochemistry Review
Wednesday: Test over chapter 6
HOMEWORK: Look at chapter 16, Thermodynamics
===================================
PROPERTIES OF SOLUTIONS
Topics to Know
Types of Solutions, Solubility Factors, Concentration Expressions, Raoult’s Law: Vapor Pressure, Non-Ideal solutions, Colloids
Concepts to Know
- Understand the properties of solutions.
- Be able to perform calculations relating to molarity, mole fraction, mass percent, and molality.
- Describe the factors that affect solubility.
- Understand the process of solution formation.
- Understand and be able to perform calculations relating to the Beer-Lambert law.
- Understand the concept of vapor pressure.
- Be able to relate changes (both quantitative and qualitative) in vapor pressure to addition of non-volatile solutes to solvents (Raoult’s Law).
- Understand and recall Raoult’s Law in terms of ideal solutions of two volatile components AND deviations from ideal behavior.
- Be able to recall and use equations relating to quantitative treatments of Boiling Point Elevation, Freezing Point Depression, Osmotic Pressure and the van’t Hoff factor.
- Be familiar with colloids and how they differ from solutions.
To Do
Read Chapter 11, pages 484-517
Ch. 11 Problem set (due February 6): 25, 31, 37, 43, 45, 48, 55, 58, 61, 64, 66, 74, 75
Videos to Watch
Defining Solutions: https://www.youtube.com/watch?v=hFmEQd3ozsY&list=PL9BA622AA8D49F627
Calculating Concentrations: https://www.youtube.com/watch?v=aZKIeFiOP34&list=PL9BA622AA8D49F627
Molality, Mass Percent, Mole Fraction: https://www.youtube.com/watch?v=71dlylG3deo&list=PL9BA622AA8D49F627
Solution Stoichiometry review: https://www.youtube.com/watch?v=7IMD0sJ3Tew&feature=c4-overview-vl&list=PL9BA622AA8D49F627
Dissociation into Ions: https://www.youtube.com/watch?v=aZKIeFiOP34&list=PL9BA622AA8D49F627
Colligative Properties: https://www.youtube.com/watch?v=aZKIeFiOP34&list=PL9BA622AA8D49F627
Schedule of Topics
January 29-30
Thursday: Types of Solutions, Solubility factors, Concentration Expressions Solutions notes
Powerpoint of all lectures: Solutions – web
HOMEWORK: Properties of Solutions I SOLUTIONS I
Friday: Colligative Properties
HOMEWORK: Properties of Solutions II SOLUTIONS II
February 2-6
Monday: Colligative Properties, Colloids
HOMEWORK: Properties of Solutions III PROPERTIES OF SOLUTIONS III
Tuesday: LAB: Analysis of Food Dyes in Beverages Dyein to Know Ya lab
HOMEWORK: Lab due Thursday, February 12
Wednesday: LAB: Analysis of Food Dyes in Beverages
HOMEWORK: Lab due Thursday, February 12
Thursday: LAB: Analysis of Commercial Bleach Analysis of Commercial Bleach
HOMEWORK: Lab due Tuesday, February 17
Friday: Homework check over bookwork, practice
HOMEWORK: Solution Free Response SOLUTIONS FREE RESPONSE
February 9-11
Monday: LAB: Analysis of Commercial Bleach Analysis of Commercial Bleach
HOMEWORK: Lab due Tuesday, February 17
Tuesday: Review for test Solutions Review
HOMEWORK: Study for test
Wednesday: Test
HOMEWORK: Labs due Thursday, Feb. 12 and Tuesday, February 17
========================================
LIQUIDS AND SOLIDS
Topics to Know
Dipole-Dipole forces, Hydrogen bonding, London Dispersion Forces, surface tension, capillary action, viscosity, crystalline solid, amorphous solid, lattice, metallic bonding, network covalent solid, molecular solid, ionic solid, vapor pressure, enthalpy of vaporization or fusion, phase diagram
Concepts to Know
- Describe characteristic properties of gases, liquids, and solids with respect to the kinetic molecular theory of matter.
- Understand the essential difference between intra and inter bonding.
- Understand the occurrence, relative strength and nature of dipole-dipole interactions, London dispersion forces and hydrogen bonds.
- Understand how solid structure influences properties.
- Understand the nature of liquids.
- Understand the concept of vapor pressure.
- Describe a phase diagram and a heating curve.
To Do
Read Chapter 10, pages 424-473
Ch. 10 Problem set (due January 28): 29, 33, 35, 40, 61, 71, 81, 84, 87, 91, 92
Videos to Watch
Phases of Matter: https://www.youtube.com/watch?v=PkAyG_the-k
Crystalline Solids: https://www.youtube.com/watch?v=D7UEIIft30g
Vapor Pressure and Phase Diagrams: https://www.youtube.com/watch?v=slREpVwWBtY
Schedule of Topics
January 16
Friday: IMFs, liquids Notes for the unit:Liquid – Solid notes
PowerPoint for all concepts: Liquids and Solids – Web
HOMEWORK: Liquids and Solids Free Response I LIQUIDS AND SOLIDS FREE RESPONSE I
January 21-23
Wednesday: Solids
HOMEWORK: Liquids and Solids Free Response II LIQUIDS AND SOLIDS FREE RESPONSE II
Thursday: Vapor pressure, changing phases, phase diagram
HOMEWORK: Liquids and Solids Free Response III LIQUIDS AND SOLIDS FREE RESPONSE III
Friday: Practice – Multiple Choice for Liquids and Solids Liquids & Solids (AP MC)
HOMEWORK: Multiple Choice for Liquids and Solids
January 26-28
Monday: Homework check over problem set, practice
HOMEWORK: Liquids and Solids Free Response IV LIQUIDS AND SOLIDS FREE RESPONSE IV
Tuesday: Review for test
HOMEWORK: Study for test
Wednesday: TEST over chapter 10
HOMEWORK: Review Solutions
=========================================
PROPERTIES OF GASES
Topics to Know
Pressure, Boyle’s Law, Charle’s Law, Gay-Lussac’s Law, Avogadro’s Law, Combined Gas Law, Ideal Gas Law, Dalton’s law of Partial Pressures, standard conditions, gas stoichiometry, KMT, velocity of gases, effusion, diffusion, Graham’s Law of Effusion, real gases versus ideal gases, van der Waals equation.
Concepts to Know
- Be able to convert between different units of pressure.
- Be able to convert between different units of temperature.
- Recall and be able to use Boyle’s law in calculations.
- Recall and be able to use Charles’ law in calculations.
- Recall and be able to use Gay-Lussac’s law in calculations.
- Recall and be able to use Avogadro’s law in calculations.
- Recall and be able to use the Combined Gas Law in calculations.
- Recall and be able to use the Ideal gas law in calculations.
- Recall and be able to use Dalton’s law of partial pressures in calculations.
- Recall the conditions that are used as standard in calculations.
- Be able to use molar gas volume in calculations.
- Understand the Kinetic Molecular theory as applied to gases.
- Understand the concept of the root-mean-square-velocity of gases.
- Understand the terms effusion and diffusion and be able to perform calculations relating to those concepts.
- Be able to discuss how real gases deviate in behavior from ideal gases.
- Understand the van der Waals equation (modified ideal gas law) in calculations.
To Do
Read Chapter 5, pages 178-216
Ch. 5 Problem set (due January 14): 33, 35, 39, 42, 44, 45, 47, 49, 51, 53, 58, 61, 66, 69, 72, 86
Videos to Watch
Pressure: https://www.youtube.com/watch?v=-us3XbO-MPs
Barometers: https://www.youtube.com/watch?v=ad2fEADrf2g
Gas Laws: https://www.youtube.com/watch?v=DSiEFv0LN3w
Ideal gas Law: https://www.youtube.com/watch?v=IGKa1ppFHz0
Molar mass of a gas: https://www.youtube.com/watch?v=wfnNjmVV0hw
Dalton’s Law of partial Pressure: https://www.youtube.com/watch?v=0MghR6h1bTM
Gas Stoichiometry: https://www.youtube.com/watch?v=DCchFZstm_w
Effusion, Diffusion, and Real Gases: https://www.youtube.com/watch?v=qjabKbRtbv8
Kinetic-Molecular Theory, Root Mean Square velocity: https://www.youtube.com/watch?v=b3sCz3cV0jQ
Schedule of Topics
January 6-9
Tuesday: Syllabus, Second semester schedule, pressure, Boyle’s Law, Charles’ Law, Gay-Lussac’s Law, and Avogadro’s Law. The PowerPoint for the three days of notes: Properties of gases – web
HOMEWORK: Gas Laws Practice, GAS LAWS PRACTICE I; Problem set due January 16
Wednesday: Ideal Gas law, Gas Stoichiometry, Calculating Density
HOMEWORK: Gas Laws Practice II GAS LAW PRACTICE II
Thursday: Dalton’s law of partial Pressures, Kinetic Molecular Theory, Graham’s Law of Effusion and Diffusion, Real vs. Ideal Gases.
HOMEWORK: Gas Laws Practice III Gas Laws Practice III
Friday: Practice Free Response for Gas laws
HOMEWORK: Gas Laws Free Response I Gas Laws Free response I
January 12-16
Monday: LAB: Determination of the Molar Mass of Volatile Liquids MM of a Volatile Liquid
HOMEWORK: Lab due Wednesday, January 21
Tuesday: LAB: Determination of the Molar Mass of Volatile Liquids
HOMEWORK: Lab due Wednesday, January 21
Wednesday: Homework check over problem set, practice
HOMEWORK: Gas Laws Free Response II, 2006 #3 and 2003 #2
Thursday: Review for test Gases – Review
HOMEWORK: Study for test
Friday: Begin Liguids and Solids content
January 20
Tuesday: TEST over chapter 5
HOMEWORK: Review liquids and solids, lab due Wednesday
=============================================
CHEMICAL BONDING
Concepts to know
- Understand that when forming chemical bonds atoms are attempting to form more stable electronic configurations.
- Understand the concept of electronegativity.
- Understand that differences in electronegativity in covalent molecules causes dipoles and some ionic character in covalent compounds
- Understand that ionic bonding and covalent bonding are at two ends of a sliding scale of bond type.
- Understand that polarization caused by small highly charged cations leads to ionic compounds exhibiting some covalent character
- Understand the concept of ionic bonding and the nature of the ionic bond.
- Understand the concept of covalent bonding and nature of the covalent bond.
- Be able to draw Lewis structures.
- Understand the concept of resonance related to Lewis structures.
- Understand the concept of formal charge related to Lewis structures.
- Be able to predict the shape of, and bond angles in, simple molecules and ions using VSEPR theory.
- Understand the concept of the coordinate covalent bond related to Lewis structures.
- Be able to predict the shapes of simple molecules and ions using Lewis structures
- Understand when molecules exhibit polarity
- Describe the formation of sigma and pi bonds and determine sigma and pi bonds in any given molecule.
- Describe covalent bond formation in terms of overlap of atomic orbitals.
- Understand and be able to identify different types of orbital hybridization.
To Do
Read Chapter 8, pages 329-381 and Chapter 9, pages 390-403
Ch. 8 Problem set (due December 9): 20, 22, 25, 27, 32, 39, 67a-e, 74, 84, 87a-e, 90, 92, 96, 100, 114
Ch. 9 Problem set (due December 9): 15, 18, 20, 21 (#67 only), 27a-f, 33
Videos to Watch
Electronegativity and Types of Bonds: http://www.youtube.com/watch?v=HKGkCbH2wxY Or http://www.youtube.com/watch?v=IGyITZmkloA&feature=c4-overview-vl&list=PL4A2CC0BDAD15594F
Polar or Dipole Moments: http://www.youtube.com/watch?v=CY6LPts8MsU&feature=c4-overview-vl&list=PL4A2CC0BDAD15594F
Lattice Energy: http://www.youtube.com/watch?v=yMSEMSsI550 OR http://www.youtube.com/watch?v=q_nf7JmB9iI&list=PL4A2CC0BDAD15594F
Bond Energy calculations: http://www.youtube.com/watch?v=ih11AH0lEZY&list=PL4A2CC0BDAD15594F
LE Bonding model and Lewis structures: http://www.youtube.com/watch?v=ks0vnq17H8s
Lewis diagrams: http://www.youtube.com/watch?v=2vDwEJs4YWs&list=PL4A2CC0BDAD15594F and http://www.youtube.com/watch?v=ajwr2rZ3m4s&list=PL4A2CC0BDAD15594F and http://www.youtube.com/watch?v=pX3tRAEAhVY&list=PL4A2CC0BDAD15594F
Lewis structures of polyatomic ions: http://www.youtube.com/watch?v=BgM5OY9U9PI&list=PL4A2CC0BDAD15594F
Exceptions, resonance, and formal charge: http://www.youtube.com/watch?v=2umwd9Z_IVs OR http://www.youtube.com/watch?v=DIjFEYNN3qg&list=PL4A2CC0BDAD15594F
Formal charge: http://www.youtube.com/watch?v=YVEvkiCh1mQ&list=PL4A2CC0BDAD15594F and http://www.youtube.com/watch?v=mbFBU2htDPk&list=PL4A2CC0BDAD15594F
VSEPR: http://www.youtube.com/watch?v=Xh5dx9zlX3I
Orbital hybridization: http://www.youtube.com/watch?v=z24942I0h-8
Sigma and Pi bonds: http://www.youtube.com/watch?v=YcSPPKESpwc
Schedule of Topics
November 19-21
Wednesday Types of Bonds, Electronegativity and bond type, dipole moments, energy effects in ionic compounds
Notes for the entire chapter
Powerpoint for the notes: Chemical Bonding One – web
HOMEWORK: Chemical Bonding handout due Friday Chemical bonding practice
Thursday Covalent bonding, LE bonding model, Lewis structures, exceptions
HOMEWORK: LE and Lewis Structures handout LE and LEWIS STRUCTURES
Friday Resonance structures, formal charge, VSEPR
HOMEWORK: Resonance and Octet Exceptions due tomorrow Resonance and Octet Exceptions
December 1-5
Monday VSEPR, molecular polarity VESPR Table
HOMEWORK: VSEPR I due tomorrow VSEPR I
Tuesday Practice – VESPR II, Chemical Bonding I Free Response I VSEPR II CHEMICAL BONDING I FREE RESPONSE
HOMEWORK: finish Free Response, due Friday, December 5
Wednesday LAB: What’s in the Bottle
Lab handout What’s in the Bottle
Powerpoint for lab – last slide is of the potential unknowns What’s in the Bottle – Web
HOMEWORK: Lab due December 12
Thursday LAB: What’s in the Bottle
HOMEWORK: Lab due December 12
Friday Hybridization
Powerpoint for lecture: Chemical Bonding II – web
HOMEWORK: VSEPR and Hybridization VSEPR and hybridization
December 8-12
Monday Practice Chemical Bonding II Free response A
HOMEWORK: Chemical Bonding II Free Response B Chemical Bonding II Free Response B
Tuesday Homework check over problem set, practice
HOMEWORK: Chemical Bonding I Free Response II due tomorrow CHEMICAL BONDING I FREE RESPONSE
Wednesday Review for test Chem Bonding Review 2014, Chem Bonding I Review – table
HOMEWORK: Study for test
Thursday Test over Chapter 8, Chemical Bonding and Molecular Structure
HOMEWORK: Study for Final AP Final Exam Review
Friday Free Response 2000, #1 and 2 given for homework to practice.
It can be found at the AP Central site: http://apcentral.collegeboard.com/apc/members/exam/exam_information/157008.html
HOMEWORK: Study for Final; Free Response 2000, #1 and 2 only.
December 15-19
Monday Review for final – homework check over exam review problems
HOMEWORK: Study for Final
Powerpoint for the review: FINAL EXAM REVIEW – Web
Tuesday Review for Final
HOMEWORK: Study for Final
Wednesday Final Exam seventh period
HOMEWORK: Multiple Choice Review, Part One, due Wednesday, January 7
========================================
ATOMIC STRUCTURE AND PERIODICITY, Chapter 7
Topics to know
Electromagnetic radiation, nature of matter, the Bohr Model, the Quantum Mechanical Model of the atom, orbital energies and shapes, electron spin, the history of the periodic table, electron configuration and orbital diagrams, periodic trends: ionization energy, electron affinity, electronegativity, atomic radii, ionic radii, family trends.
Objectives for Unit
1. Understand the Bohr model of the atom.
2. Understand how line emission spectra are formed.
3. Appreciate that the electron can be considered to have wave like properties as well as particle type properties.
4. Understand and use equations that relate the Energy, frequency, speed and wavelength of waves.
5. Understand the concept of electrons in shells and the use of quantum numbers.
6. Understand the use of the terms s, p, d and f and their use in orbital notation.
7. Recall and understand the rules for filling orbitals and determining electronic configuration, including the Pauli Exclusion Principle, Hund’s rule of maximum multiplicity and notable exceptions. (No need to memorize the exceptions – but be able to explain them.)
8. Be able to construct the electronic configuration of the elements using the s, p and d and f notation as well as the shorthand configuration using the noble gases
9. Be able to construct the electronic configuration of simple ions (including d block ions).
10. Recall the shapes of the s, p and d orbitals.
11. Recall that orbitals are electron probability maps.
12. Be able to describe electronic configurations using the electrons in orbital diagram (box) notation.
13. Recall the meanings of the terms paramagnetic, diamagnetic and isoelectronic.
To Do
Read Chapter 7, pages 274-319
Ch. 7 Problem set (due November 10): 31, 33, 37, 41, 44, 45, 49, 51, 57, 58, 67, 69, 73, 80, 85, 87, 89, 93, 95, 99
Videos to Watch
Planck’s Constant: http://www.youtube.com/watch?v=lTnpb9YPrQs
Photoelectric Effect: http://www.youtube.com/watch?v=0qKrOF-gJZ4
Atomic Emission Spectra: http://www.youtube.com/watch?v=qEg8be6zQgA
Bohr Model: http://www.youtube.com/watch?v=R7OKPaKr5QM OR http://www.youtube.com/watch?v=45KGS1Ro-sc
The Wave Mechanical Model of the Atom: http://www.youtube.com/watch?v=1bpG1lEjJfY
Paramagnetism and diamagnetism: http://www.youtube.com/watch?v=dVWtvG9ztMQ
Quantum numbers: http://www.youtube.com/watch?v=oK6K68ADKDA&feature=c4-overview-vl&list=PLD16C5E9C8EA3D621
Writing Quantum numbers for electrons: http://www.youtube.com/watch?v=a4fklbnHTYY&list=PLD16C5E9C8EA3D621
Electron Configuration and Orbital Diagrams: http://www.youtube.com/watch?v=9xHRV48oC80&list=PLD16C5E9C8EA3D621
Periodic Trends: http://www.youtube.com/watch?v=G3qbooMh6Fc
Schedule of Topics
October 31
Friday Electromagnetic Radiation through Bohr Atomic Structure-Periodicity Notes
HOMEWORK: Atomic Structure Practice handout Atomic Structure Practice
November 3-7
Monday Quantum Mechanical Model through Pauli, Hund, and Quantum Numbers
HOMEWORK: Atomic Structure Practice II handout Atomic Structure Practice II
Tuesday Determining Quantum Numbers, Electron Configuration, and Orbital Diagrams for atoms and ions.
HOMEWORK: Quantum Numbers, Electron Configuration, and Orbital Diagrams for Atoms and Ions QNumbers EConfig Orbital
Wednesday Periodic trends lab Trending – Periodicity lab
HOMEWORK: Lab due November 13
Thursday Periodic trends lab
HOMEWORK: Lab due November 13
Friday Periodic Trends
HOMEWORK: Atomic Structure and Periodicity Free Response Free response 1980 1987 1997
PowerPoint for the notes: Atomic Structure and Periodicity – web
November 10-14
Monday Homework Check – chapter 7, Practice
HOMEWORK: Periodic Trends Free Response II (Ap Free Response – 2005 7cd and 2006 8abcd; download from AP Central website.)
Tuesday Review for test
HOMEWORK: Review and study for test
Wednesday Test
HOMEWORK: Review bonding
Thursday Organic Nomenclature
HOMEWORK: Work on packet
Friday Organic Nomenclature
HOMEWORK: Work on packet
November 17-18
Monday Organic Nomenclature
HOMEWORK: Work on packet
Tuesday Organic Quiz
HOMEWORK: Review chapters 8-9
===============================================
ELECTROCHEMISTRY, Chapter 17
Concepts to know
1. Recall the definition of oxidation and reduction in terms of electrons.
2. Understand and recall the definition of standard electrode potential.
3. Understand and recall how to construct a cell diagram (line notation) and draw a diagram (picture) of the apparatus needed.
4. Recall the conditions that standard electrode potentials are measured under.
5. Understand the nature and purpose of a salt bridge.
6. Be able to predict the likelihood or otherwise of chemical reactions using standard electrode potentials and understand how those predictions may not prove to be accurate.
7. Understand and use the Nernst equation.
8. Understand the relationship between Gibbs free energy, equilibrium constant and Ecell, and be able to perform related calculations.
9. Understand electrolysis and be able to perform quantitative calculations relating to it.
To Do
Read Chapter 17, pages 790-828
Ch. 17 Problem set (due October 29): 25, 27, 29, 31, 36, 37, 45, 51, 52, 55, 57, 65, 69, 71, 77, 79
Videos to Watch
Galvanic Cells: https://www.khanacademy.org/science/chemistry/oxidation-reduction/v/galvanic-cells
Galvanic Cells: http://www.youtube.com/watch?v=4NkV1VRNYpM
Good short electrochemical cell: http://www.youtube.com/watch?v=A0VUsoeT9aM
Standard Reduction Potential: http://www.youtube.com/watch?v=CjBDp8kqqbI
Gibbs Free Energy, Electrical Work, and Cell Potential: http://www.youtube.com/watch?v=IPMhVBCmFpY
Nernst and Concentration Dependency: http://www.youtube.com/watch?v=BuE9XDgxTkE
Schedule of Topics
October 9-10
Thursday: Solutions Free Response
HOMEWORK: finish free response
Friday: Look at the solutions to the Free Response
HOMEWORK: NONE!
October 13-17
Monday: No school!
Tuesday: Galvanic Cells, standard reduction potentials, line notation
HOMEWORK: Electrochemical Cell Practice Electrochemical Cell Practice
Here are the notes for the chapter: Electrochemistry Notes
Standard Reduction Potential Chart – text: Text Std Reduction Pot Chart and AP Central: AP Std Reduction Pot Chart
Wednesday: Cell potential, Electrical work, and free energy
HOMEWORK: Electrochemistry Free Response and Practice Electro FR and Practice
Thursday: Effect of concentration, Nerst Equation, Applications of galvanic cells
HOMEWORK: Nernst and Concentration Nernst and Concentration
Friday: Electrolysis, Electrolytic cells, applications
HOMEWORK: Electrolysis Practice Electrolysis Free Response
Also do the pre-lab for the lab Monday: Galvanic Cell – pre lab
October 20-24
Monday: Galvanic Cell lab
HOMEWORK: Lab due October 28
Lab Directions:Galvanic Cell lab directions
Lab Data and Results sheet: Galvanic Cell lab data table
Tuesday: Galvanic Cell lab
HOMEWORK: Lab due October 28
Wednesday: Practice – AP Free Response 2000, #2 and 2004, #6
HOMEWORK: Electrochemistry Practice
Thursday: Electrolysis Lab Electrolytic Cell lab
HOMEWORK: Electrolysis lab due November 3
Friday: Electrolysis Lab
HOMEWORK: Electrolysis lab due November 3
October 27-29
Monday: Homework Check over Ch. 17 problem set
HOMEWORK: Electrochemistry Free Response 2005, #8d
Tuesday: Review for Electrochemistry Test Electrochem review
HOMEWORK: Study for test!
Wednesday: Electrochemistry Test
HOMEWORK: Gavlanic Cell Lab due tomorrow
Monday, November 3: Electrolytic Cell lab due
==================================
Types of Chemical Reactions and Solution Stoichiometry
Topics to Know
Water the common solvent, Strong and weak electrolytes, Composition of solutions, Solution Stoichiometry, Types of Chemical Reactions: Precipitation, net ionic, acid-base, oxidation-reduction
To Do
Read Chapter 4, pages 126-177
Ch. 4 Problem set (due October 6): 22, 24, 25, 28, 30, 38, 40, 47, 50, 60, 62, 63, 65, 68, 72, 74abc,76, 77
Videos to Watch
Practicing Precipitation and acid-base stoichiometry:
Writing Net ionic equations:
Writing Redox Reactions:
Schedule of Topics
September 23-26
Tuesday: Water the common solvent, Strong and weak electrolytes, Composition of solutions (molarity, volume, ion, dilution calculations)
The unit notes: Soln – Ch. 4 – notes
HOMEWORK: Solution Calculations handout due Friday; problem set due October 6
Solutions Calculations handout: Soln Calculations
Wednesday: Types of Chemical Reactions: Precipitation, net ionic
HOMEWORK: Solubility and Net Ionic Equation Practice due Friday Solubility and net ionic practice
Here are the notes and practice I gave out to first year chemistry students on net-ionic equations: Net ionic notes – gifted
Thursday: Work on Solution Calculations handout and Solutions Multiple Choice handout due Friday
Solutions Multiple Choice: Solutions Multiple Choice
Friday: Solution stoichiometry
HOMEWORK: Solution Stoichiometry handout due Monday Solution Stoichiometry
September 29 – October 3
Monday Acid-base reactions, Neutralization reactions, titrations,
HOMEWORK: Acid Base Reactions handout due Tuesday Acid Base Reactions
Tuesday Oxidation Reduction reactions
HOMEWORK: Redox I handout due Friday, Redox I Prelab due Wednesday
Prelab sheet: Test Tube Mystery prelab
Notes given to first year chemistry students on redox equations: Redox notes-gifted
Wednesday Lab: Test Tube Mystery
HOMEWORK: Lab due Thursday, October 9
Here are the lab sheets: Test Tube Mystery lab – student
Thursday Lab: Test Tube Mystery
HOMEWORK: Lab due Thursday, October 9
Friday Practice Redox
HOMEWORK Redox II handout due Monday Redox II
October 6-8
Monday HW CK: Ch. 4 problem set, Practice
HOMEWORK: handout due Tuesday Types of Rxn and Soln Stoich Multiple Choice
Tuesday Review for test Ch 4 Review
HOMEWORK: study for test
Wednesday Test
===================================
CHEMICAL EQUILIBRIUM
Topics to know
Chemical Equilibrium; equilibrium constant, K; equilibrium positions; Equilibrium Expressions involving pressure; ICE calculations; Reaction Quotient, Q; Le Chatelier’s Principle.
To Do
Read Chapter 13, pages 579-611
Ch. 13 Problem set (due September 18): 17, 18, 20, 21, 24, 26, 27, 29ab, 33, 35, 38, 40, 41, 46, 48, 57, 58, 61, 63.
Videos to Watch
EQ position and constant: http://www.youtube.com/watch?v=0bIjEc89bI0
EQ Pressure and Heterogeneous Equilibria: http://www.youtube.com/watch?v=B6HEnMFQJGI
ICE Table – normal: http://www.youtube.com/watch?v=z6nqtyOQ6e8&list=PL8C08CCD559540F12
ICE Table with small K: http://www.youtube.com/watch?v=TnSTCBchSfs&list=PL8C08CCD559540F12
ICE table with quadratic equation: http://www.youtube.com/watch?v=tT-2xk9ZG_A
Schedule of Topics
August 29
Friday: chemical equilibrium, law of mass action, heterogeneous equilibria
Here are the notes for the whole unit: EQnotes-AP
HOMEWORK: due Wednesday – K equation practiceI
September 3-5
Wednesday: Equilibrium expressions involving pressure; calculating K from Kp
Here is the handout we did at the beginning of class Wednesday: K equation pressure practice
HOMEWORK: due Thursday – Equilibrium Exercise
Thursday: Reaction Quotient, Q
HOMEWORK: due Friday – Equilibrium Exercise II
Friday: Quiz over K, Kp, Q, Practice
Here is the handout we did at the beginning of the period: Reaction Quotient practice
HOMEWORK: due Monday – 2000 Free response
September 8-12
Monday: ICE calculations
HOMEWORK: due Thursday – ICE EXERCISES I
Tuesday: Lab – Chemical Equilibrium: Finding a Constant, Kc
HOMEWORK: Lab due Wednesday, September 17
Wednesday: finish lab
HOMEWORK: Lab due Wednesday, September 17
Thursday: Practice
HOMEWORK: due Friday – ICE EXERCISES II
Friday: Le Chatelier’s Principle
HOMEWORK: due Wednesday – LeChatelier Practice I
September 15-19
Monday: SLO (This pretest date is subject to change based on the County calendar and when the SLO is made available.)
Tuesday: Lab – Can We Make the Colors of the Rainbow?
HOMEWORK: lab due Wednesday, September 24
Wednesday: finish lab
HOMEWORK: lab due Wednesday, September 24
Thursday: Homework Quiz over problem set; practice
HOMEWORK: due Thursday – Le Chatelier Free Response
Friday: Review for test
Review packet: Chem Eq Review
September 22
Monday: Test over chapter 13
==========================================
Introduction, Matter, Atoms, Molecules, and Ions, and Stoichiometry
Topics to Know
Chapter 1: scientific method, units, measurements, significant figures, scientific notation, accuracy and precision, dimensional analysis, matter, classes (element, compound, mixture, solution), separation techniques.
Chapter 2: fundamental laws, atomic theory, atomic structure, ions, periodic table basics, nomenclature.
Chapter 3: mole, molar mass, percent composition, empirical and molecular formulas, balancing equations, stoichiometry, limiting reactant.
Videos to Watch
Significant Figures: http://www.youtube.com/watch?v=eCJ76hz7jPM
Dimensional Analysis: http://www.youtube.com/watch?v=DsTg1CeWchc
Separation Techniques for Matter: http://www.youtube.com/watch?v=DqLKPmGyXbE
Fundamental Laws: http://www.youtube.com/watch?v=QiiyvzZBKT8
Atomic Theory: http://www.youtube.com/watch?v=eXdWlnBlncM
Periodic Table Basics: http://www.youtube.com/watch?v=E-Mor6RbR_s
Nomenclature: http://www.youtube.com/watch?v=9XUsOLaz3zY&list=PLDBEA95CB871EF1C4
and http://www.youtube.com/watch?v=16agvZ8K2eM
The Mole: http://www.youtube.com/watch?v=tEn0N4R2dqA
Problem Set to Do – Due Wednesday, August 27
Chapter 1: 20, 21, 24, 26, 28, 32, 33, 37, 43, 54, 63, 70, 73, 76, 82
Chapter 2: 17, 18, 22, 24, 25, 28, 44, 54, 61, 64, 65, 68, 78, 80, 82
Chapter 3: 22, 25, 28, 36, 39, 44, 54, 62, 68ab, 69, 82, 86, 91, 94, 102, 106
Schedule of Topics
August 11-15
Monday: Syllabus, Safety Contract, Communication form
Gifted Syllabi – AP – Brim2014
Tuesday: Pretest
Wednesday: Metric units, measurements, significant figures, scientific notation
Powerpoint on first week info: Chapters 1-2.7-web
First year chemistry notes on metric measurements (Metric Notes), and significant figures and scientific notation (SciNote-SigFig Notes).
Thursday: Accuracy and precision, dimensional analysis.
First year chemistry notes on dimensional analysis (Dim-Ana-practice).
Friday: Atomic theory, atomic structure, periodic table basics.
First year chemistry notes on atomic theory and structure: Atom Theory notes
August 18-22
Monday: Nomenclature
First year chemistry notes on chemical nomenclature: Nomenclature notes
Tuesday: Quiz over first week topics; work on problem set due next Wednesday.
Wednesday: LAB: How much of a penny is zinc?
Penny Lab: Properties of a Penny Lab
HOMEWORK: Lab due Wednesday
Thursday: Finish Penny lab; Mole, molar mass, percent composition, empirical formula, molecular formula practice
Handout: Mole thru molecular formula review
Friday: Mole, molar mass, percent composition, empirical formula, molecular formula
Notes on Empirical Formula through Percent Yield: Stoichio Notes
Powerpoint on Mole through Percent Yield: mole thru Stoichio
Free response 1991B and 1995: Stoichiometry1991B-1995B
August 25-29
Monday: Stoichiometry and limiting reactants, percent yield
Tuesday: LAB: Paper Chromatography
Paper Chromatography lab: Chromatography lab
Wednesday: Problem set due, quiz over homework
HOMEWORK: Stoichiometry Practice Stoichiometry Test Practice
Thursday: Review for test
Review packet: Ch 1-3 review
Friday: Start Equilibrium
Tuesday, September 2: Test over chapters 1-3